A new crystallographic technique is set to transform scientists’ ability to observe how molecules work. A research paper, published by a team of researchers led by Professor Arwen Pearson in the journal Nature Methods describes a new way of doing time-resolved crystallography, a method that researchers use to observe changes within the structure of molecules.
Although fast time-resolved crystallography (Laue crystallography) has previously been possible, it has required specifically configurated instrumentation that is only available at three sites worldwide. Only a handful of proteins have been studied using the traditional technique. The new method will allow researchers across the world to carry out dynamic crystallography and is likely to provide a major boost in areas of research that rely on understanding how molecules work, such as the development of novel smart materials or new drugs. Understanding how structure and dynamics are linked to function is key to designing better medicines that are targeted at specific states of molecules, helping to avoid unwanted side effects.
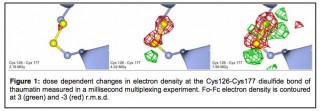
The picture shows the progressive breaking of a disulphide bond recorded using the new method. Illustration: Arwen Pearson
“A time-resolved structure is a bit like having a movie for crystallographers,” said Professor Arwen Pearson, who led the team at the University of Leeds, where the method was developed; since May 2014, Arwen Pearson is Professor at CUI. “Life wiggles. It moves about and, to understand it, you need to be able to see how biological structures move at the atomic scale. This breakthrough allows us to do that.”
Traditional X-ray crystallography fires X-rays into crystallised molecules and creates an image that allows researchers to work out the atomic structure of the molecules. A major limitation is that the picture created is the average of all the molecules in a crystal and their motions over the time of an experiment.
Dr Briony Yorke, the lead researcher on the project and now postdoc in Hamburg, said: “A static picture is not very helpful if you want to observe how molecular structures work. It is like trying to find out how a car works without being allowed to run the engine. You can look at the spark plugs and the piston and maybe take a guess at how it is going to function, but it is hard to really understand something without seeing it in action.”
How moving pictures are built up
The existing method of getting around the problem could be compared to the laborious process of making an animated film. Scientists “synchronise” a set of molecules in an identical state and then activate, or “pump”, the changes in the molecules. They take a crystallographic snapshot of the structure after a set time. The researchers then have to begin the whole experiment again: synchronising the molecules, “pumping” them and then taking a snapshot a bit later in the process. Slowly, they build up a moving picture.
This pump-probe approach was first proposed in Nobel Prize winning research by the British chemist George Porter in the 1940s and has had a huge impact on our understanding of chemistry. However, a major limitation of the “pump-probe” approach for crystallographic experiments is that the snapshots are only “exposed” for a moment (often as short as 100 million millionths of a second) in order to capture the molecular movements. That means there is very little time to deliver enough light to create a crystallographic image. There are only three “synchrotrons”, large X-ray generators, in the world that deliver the necessary beam.
The new method uses clever maths (a Hadamard Transform) to open up the field to much less powerful synchrotron “beamlines”, advanced laboratories that scientists use to harness powerful synchrotron light for crystallography and other techniques. As in Porter’s method, in the new approach researchers synchronise their molecules and activate them. However, they then make a series of crystallographic “probes” of the moving structures using a pattern of light pulses. These pulses build up a single crystallographic image—a bit like a long exposure photograph. The researchers then repeat the experiment using a different pattern of light pulses and create a different “long exposure” image. This process is repeated until all of the pulse patterns created using a mathematical formula have been completed.
No need for clear snapshots
Each of the “long exposure” images created from the pulse patterns is blurred, but the differences in the images and between the pulse patterns that created them allow researchers to extract a moving picture of the molecules’ changing structures.
Professor Pearson said: “The great thing about this method is that we don’t need the clear snapshots, and therefore the very strong light, required by the Porter method. This is a completely new way of doing a time-resolved experiment and overcomes many of the current limitations.”
Original Publication:
Briony A Yorke, Godfrey S Beddard, Robin L Owen, and Arwen R Pearson
“Time-resolved crystallography using the Hadamard Transform”
Nature Methods (2014)
DOI: 10.1038/nmeth.3139
Contact:
Prof. Arwen Pearson
The Hamburg Centre for Ultrafast Imaging (CUI)
Arwen.pearson@cfel.de
040/8998-6650
Dr. Briony Yorke
The Hamburg Centre for Ultrafast Imaging (CUI)
Briony.yorke@cfel.de
040/8998-6655

