Vitamin transporters are responsible for the uptake of essential nutrients in bacteria. A team of scientists led by CUI member Prof. Henning Tidow (Universität Hamburg) has determined the structure of a thiamine transport protein – an important milestone in membrane transport research and for drug discovery in the long run. The team reports its findings in the recent issue of the journal Cell Chemical Biology with an artistic version as cover image.

ECF transporters are proposed to function via a unique toppling mechanism. The cover image was created by Oliver Hoeller: www.oliverhoeller.com
Bacteria are found everywhere, in the air, soil, water, and inside your body and on your skin. Some bacteria are used to supply products that improve human life. Because of their ability to quickly grow and the relative ease with which they can be manipulated, bacteria are the workhorses for the fields of molecular biology, genetics and biochemistry.
Like any other organism, bacteria require small amounts of organic compounds from the diet. “Many bacteria, however, cannot synthesize essential vitamins but rely on the uptake from their environment,” Tidow explains. The machines performing this task are so-called energy-coupling factor (ECF) transporters, a family of integral membrane proteins which are proposed to function via a unique toppling mechanism. In their research project, the scientists concentrated on one of the two subgroups by which the protein family is classified: the more obscure, group I ECF transporters contain a dedicated scaffold protein (T) and substrate-binding protein (S). “We were familiar with S- and T-components, but the molecular details of their interaction or structures have so far been unknown”, says CUI postdoc Inokentijs Josts, first author of the study.
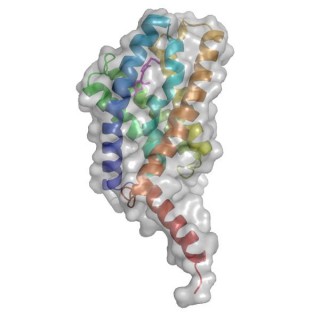
Structure of YkoE with bound thiamine. Credit: Henning Tidow, Universität Hamburg
By way of lipidic-cubic phase X-ray crystallography complemented by coarse-grained molecular dynamics simulations and fluorescence spectroscopy, the scientists determined the structure of a thiamine-bound group I substrate-capture (S-) component (YkoE) in a lipidic environment. The structure analysis revealed essential differences between YkoE and the better characterized group II ECF S-components. Thus it uncovered how group I S-components can diverge from other group I and group II ECF transporters.
Tidow: “We determined the crystal structure and gained insight into mechanistic details of the interaction between S- and T-components. This will help us to unravel the transport mechanism and dynamics used by this unique transporter family that exists in many pathogenic bacteria and may thus constitute a potential target for antibiotic development.”
Citation:
Josts I., Hernandez A., Andreeva A., Tidow H.
“Crystal structure of a group I ECF vitamin transporter S-component in complex with its cognate substrate”
Cell CHemical Biology (23) 7, 827–836 (2016)
DOI:10.1016/j.chembiol.2016.06.008

