Using high-resolution electron microscopy, a group of scientists around CUI Professor Horst Weller (Universität Hamburg) has succeeded in observing ion-exchange processes in nanocrystals. The team reports its observations, which will help to design nanomaterials for biomedical applications and optoelectronics, in the journal Angewandte Chemie.
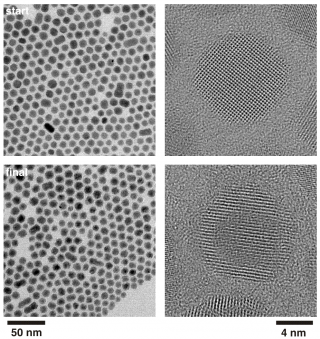
Electron microscopy shows, how the core of the crystal changes during the exchange process. Credit: Weller/Kornowski, Universität Hamburg; Wiley
Recent developments in the synthesis of nanocrystals have enabled the production of many technologically important crystalline materials in nanometer sizes. “Despite those successes, several shapes and compositions can still not be achieved directly,” says CUI Professor and team member Holger Lange (Universität Hamburg). “This is especially true for colloidal nanocrystals with a photoluminescence in the near-infrared window of biological tissue,” the scientist explains. Tissue is almost transparent in the near-infrared spectral range, so that luminescent nanocrystals can be followed easily when used as markers – for example on their way towards a tumor. Accordingly, such nanocrystals are of great interest.
A well-established method for producing such complicated combinations of materials is to tune the properties of the material via an ion-exchange process. When experimenting with nanocrystals, for example, scientists use the cation-exchange to tune the luminescent properties without altering the nanocrystal’s shape.
Horst Weller: “This process is unique to nanomaterials and, despite being regularly used, not fully understood. For example, how can ion-exchange processes occur in nanocrystals without changing the size and the shape, and why is the ion transport much faster than in classical interdiffusion processes in macrocrystalline solids?”
The scientists have now investigated these processes at the molecular level. Using high-resolution electron microscopy and kinetic experiments for several model reactions, they observed a diffusion process that proceeds exclusively through the interstitial lattice positions. This process is followed by a “kick out” to remove individual ions from lattice sites without the formation of vacancies. Weller: “We are very happy about our observations, as this fundamental mechanism has not yet been discussed with regard to nanocrystalline systems.”
Citation:
Bothe C., Kornowski A., Tornatzky H., Schmidtke Ch., Lange H., Maultzsch J. and Weller H.
Solid-State Chemistry on the Nanoscale :Ion Transport through Interstitial Sites or Vacancies?
Angewandte Chemie International Edition, Volume 54, Issue 47 Pages 14183–14186 (2015)
DOI: 10.1002/anie.201507263

