Together with colleagues from Brazil and China, Hamburg scientists working on DESY’s X-ray light sources have developed a promising approach for outsmarting hospital germs that are resistant to antibiotics. Instead of attacking such MRSA bacteria directly, the scientists meddle with a metabolic pathway that is essential to the survival of the germs. As a result, the bacteria produce a useless variant of the vitamin B1, and in the absence of functional vitamin B1 the germs die. The team surrounding CUI member Christian Betzel, a professor at Universität Hamburg, and Carsten Wrenger, a professor at the University of Sao Paulo, is presenting its findings in the journal Scientific Reports.
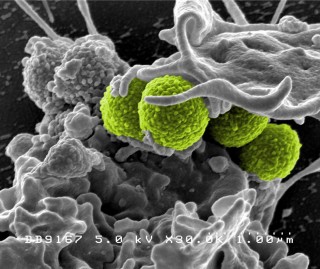
Four green-colored, spheroid-shaped, methicillin-resistant Staphylococcus aureus (MRSA) bacteria, as they were in the process of being enveloped by a much larger human white blood cell. Credit: National Institute of Allergy and Infectious Diseases (NIAID)
Resistance to antibiotics is a growing problem in the field of healthcare. More and more strains of bacteria are becoming immune to certain antibiotics – they learn to adapt to the attacks and are no longer vulnerable to them. As a result, the most important weapon against bacterial infection is threatening to be dulled. So-called methicillin-resistant Staphylococcus aureus bacteria (MRSA) are often immune to all the usual types of antibiotics and can only be treated using emergency and fall-back drugs. Initially, MRSA only occurred in hospitals, where resistances develop more easily due to the high incidence of germs and antibiotics; but in the meantime MRSA infections are also being observed outside healthcare institutions.
In the search for new agents with which to fight the resistant germs, Betzel and Wrenger’s team tried a new approach. “Classical drugs block a particular function of the bacterium,” explains Betzel. “In such cases, the bacteria can come up with a way of by-passing the blockade, which makes them resistant to that particular drug.” Instead, the researchers cleverly interfere with the vitamin B1 pathway of the staphylococci, without actually blocking it. The bacteria have to manufacture this vital vitamin themselves. At the synchrotron beam line P14 of the European Molecular Biology Laboratory (EMBL) at DESY’s X-ray light source PETRA III, the scientists determined the precise atomic structure of one of the enzymes involved in this process. The scientists then “fed” this enzyme with a custom-made, seemingly useful ingredient; however, the so-called substrate is slightly modified compared with the natural version, so that the form of the vitamin B1 produced is in fact useless.
“By doing this, we trick the organism,” explains Betzel. “We give it something that it believes it needs – but in a slightly modified form so that ultimately it is unable to use it. Ideally, the bacterium won’t notice what is wrong, because the vitamin B1 pathway continues to work as it should.” The researchers believe that this vitamin is particularly suitable for their approach, for two reasons. “The vitamin B1 pathway is absolutely essential. There are virtually no alternatives,” says CUI member Markus Perbandt, assistant professor at Universität Hamburg and one of the co-authors of the study. And on top of this: “Human beings do not have a similar enzyme. That is extremely important in order to avoid cross reactions.”
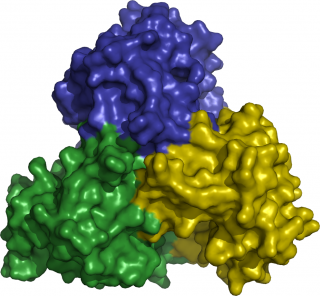
The enzyme ThiM forms a trimer. Credit: Christian Betzel/Universität Hamburg
But what must the ideal substrate look like in order for the bacteria to accept it? To find that out, the scientists used the DESY X-ray light sources to examine the atomic structure of the enzymes involved. “Six enzymes are involved in the vitamin-B1 pathway. Four of them have already been analysed,” reports Betzel. “The most interesting of these is an enzyme by the name of ThiM. We only have to alter two atoms in the substrate we ‘feed’ to this enzyme, in order to render it useless.” ThiM is a so-called trimer, meaning that it consists of three individual ThiM molecules, which form a complex. “The trimer therefore has three active sites, each located on the interface between the three molecules,” explains Betzel.
“Once you know the precise structure of the active sites, you can specifically develop a useless substrate,” says Perbandt. But the enzyme must not only use the wrong substrate; it should also prefer it to the right one, which is also available. To achieve this, the researchers make their fake substrate more chemically attractive by attaching certain molecular groups to it. “It’s a bit like offering the bacteria a piece of chocolate next to a piece of dry bread,” says Betzel. “You can only do this if you know the precise atomic structure of the enzyme you are targeting.” Medical scientists refer to such agents as prodrugs. Prodrugs only become pharmacologically active once they have been metabolised inside an organism.
“Of the twelve original candidate drugs, three have proved to be promising,” reports Perbandt. “These are now being tested on cell cultures.” Whether this will eventually lead to a new treatment, is not yet certain, but the approach of custom-designing an active agent by knowing the precise atomic structure of a biomolecule is not only suitable for medication used for treating MRSA. “This new method of structure-based drug development is a promising means of fighting other pathogens too,” says Perbandt. And the method has additional advantages: “Structure-based drug development not only saves money, but also greatly reduces the need for animal testing.”
Citation:
Drebes J.
“Structure of ThiM from Vitamin B1 biosynthetic pathway of Staphylococcus aureus – Insights into a novel pro-drug approach addressing MRSA infections”
Scientific Reports, 2016
DOI: 10.1038/srep22871

