An international team of researchers led by CUI member Prof. Henning Tidow of Universität Hamburg has found a new method for analyzing membrane proteins. The team made use of the special nature of a hydrogen isotope: deuterium labelled ‘stealth carrier’ nanodiscs (sND) are effectively invisible to low-resolution neutron diffraction and enable structural studies of integrated membrane proteins in a lipidic native-like solution environment. The team reports its findings in the recent issue of the journal Structure.
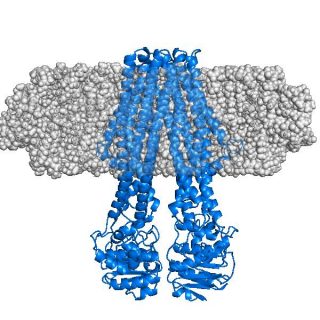
The concept of stealth carrier nanodiscs: the integral membrane protein MsbA (blue) is embedded in a semi-transparent sphere (grey) to indicate invisibility of the nanodisc to neutron radiation in SANS experiments. Illustration: Tidow, Universität Hamburg
Integral membrane proteins (IMPs) play central roles in cellular life: they are essential for the transport of matter and signals across cell membranes and are key targets for drug development. Detailed knowledge of their structures and of the dynamics of different states is therefore highly desirable. However, structural studies of IMPs are complicated by the fact that many membrane proteins suffer loss of stability, activity and function outside a lipid environment.
In this situation, nanodisc lipid bilayer scaffolds have emerged as an invaluable tool for solution-state studies of IMPs in a native-like lipid environment. Tidow: “While solution scattering techniques are highly appropriate for the study of these IMP/nanodisc systems, the complex multi-contrast scattering contribution of the scaffold disc makes data analysis and structural analysis of the incorporated IMP extremely challenging.”
Tidow and his team have now made use of sND technology to incorporate a membrane protein – the ATP-binding cassette (ABC) transporter protein MsbA – into stealth carrier nanodiscs. The effective invisibility of the carrier system enabled the team to obtain high quality small-angle neutron scattering (SANS) data from MsbA in a lipid environment. The SANS experiments were accompanied by synchrotron small-angle X-ray scattering (SAXS) measurements.
Thus the scientists could obtain structural models of different conformational states of MsbA in solution. This methodology can be applied to other classes of integral membrane proteins and paves the way for low-resolution structure determination of IMPs in solution using both ab initio and rigid body analysis approaches.
The research was carried out in close collaboration between scientists of Universität Hamburg, EMBL Hamburg, Malmö University, ILL Grenoble and MLZ Munich. Text: CUI
Citation:
Josts I. et al.
“Conformational states of ABC transporter MsbA in a lipid environment investigated by small-angle scattering using stealth carrier nanodiscs”
Structure 26, 8 (2018)
DOI: 10.1016/j.str.2018.05.007

